
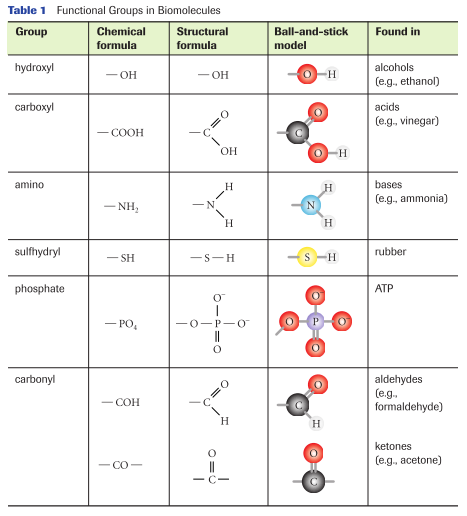
IR analyses of the AC samples were performed to detect the vibration frequency changes after chemical treatment using FTIR spectroscopy (FTIR 6700 NEXUS Nicolet, USA). The changes of element content on AC before and after modification were analyzed by elemental analyser (EURO EA3000, Italy). The reusability of carbons and the feasibility of Cu (II) recovery were also investigated. Adsorption and regeneration experiments, in combination with characterization analysis such as elemental analysis, Fourier transform infrared (FTIR) spectrometry, Boehm titration method and X-ray photoelectron spectroscopy (XPS) studies, were carried out to elucidate the role of functional groups on the carbons participating in the adsorption process. In this study, the functional groups on walnut shell-derived carbon were modified without significantly changing its pore structures, and their contribution to the copper removal was investigated. The blockage of a given functional group is to prevent its participation in the adsorption process, so the contribution of some certain functional groups in metal ion removal can be evaluated ( Marín et al. However, not much information is available on clarifying the contribution of the physical and various functional groups.Ĭhemical treatment is reported to allow the blockage of certain functional groups on the biomass ( Liu et al. Surface modifications of AC have been recognized as effective approaches to enhance the metal ion uptake from aqueous media by increasing the metal ion binding sites ( Yantasee et al.

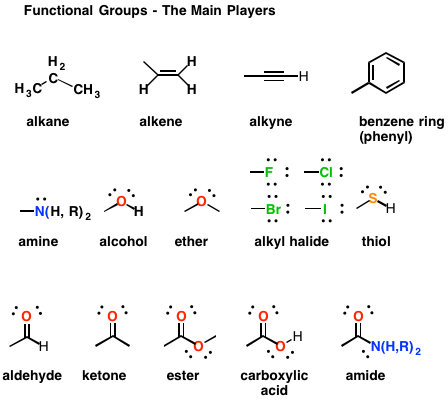
Previous research revealed oxygen and nitrogen containing functional groups would improve the uptake capability of AC towards metal ions, such as Cu (II), Cr (VI) ( Sun et al. (2004) functionalized AC with amine, which resulted in a high and rapid adsorption for copper. (2011b) modified an adsorbent with ethylenediaminetetraacetic acid, leading to an increased number of carboxyl groups on the adsorbent, resulting in a higher affinity for lead. found that introducing S −, Cl − and N − containing ligands to an AC surface would increase its adsorption capacity toward Hg ( Zhu et al. Further efforts have also been made to study the carbon's surface chemical properties in order to enhance their affinity for metal ions ( Song et al.
Corncob based ACs with a uniform micropore size (close to 2 nm) showed excellent removal capacity towards many kinds of dyes, chlorophenol and phenol ( Tseng & Tseng 2005). Micropores with sizes less than 7 nm were reported to govern the performance of AC on volatile organic compounds and benzene removal at low concentration ( Lillo-Ródenas et al. Up to now, many studies have been carried out to investigate the influence of the pore structures on the carbon's adsorption capacity ( Lu & Sorial 2004 Lillo-Ródenas et al. The adsorption capacity of AC is largely dependent on its pore structure and surface chemistry. The regeneration of copper-loaded AC and the recovery of copper were also studied to evaluate the reusability of the oxidized AC.
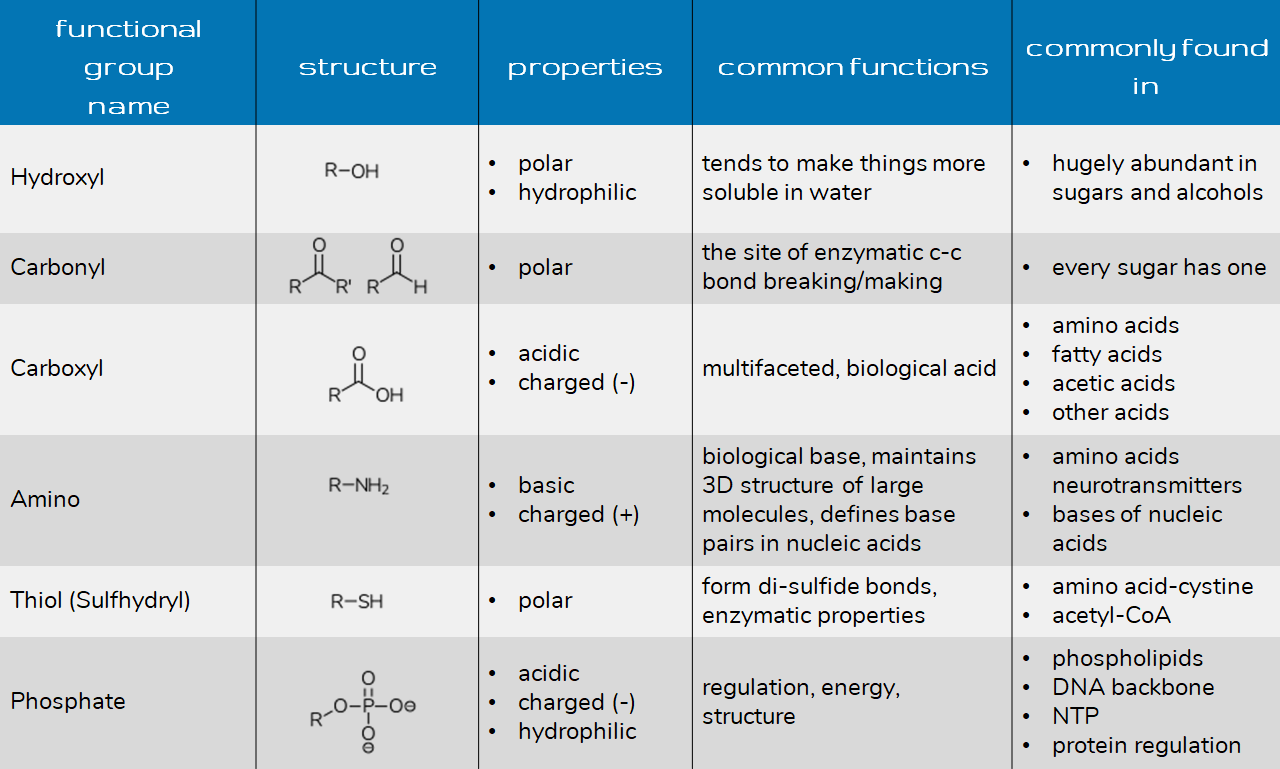
Some other types of interactions, like complexation, were also proven to be involved in the adsorption process, while physical force was found to play a small role in the copper uptake. Ion-exchange was identified to be the dominant mechanism in the copper uptake by AC. Among various surface functional groups, the oxygen-containing group was found to play a critical role in the copper uptake, and oxidation is the most effective way to improve Cu (II) adsorption onto AC. The results demonstrated that the functional groups on AC played an important role in copper uptake. The equilibrium and the kinetics of copper adsorption onto AC were studied. The structural and chemical heterogeneity of the produced carbons are characterized by Fourier transform infrared spectrometry, X-ray photoelectron spectroscopy, Boehm titration method and N 2/77 K adsorption isotherm analysis. The surface chemistry of the prepared AC was modified by introducing or blocking certain functional groups, and the role of the different functional groups involved in the copper uptake was investigated. In this study, activated carbon (AC) was prepared from walnut shell using chemical activation.


 0 kommentar(er)
0 kommentar(er)
